Cardiac Safety
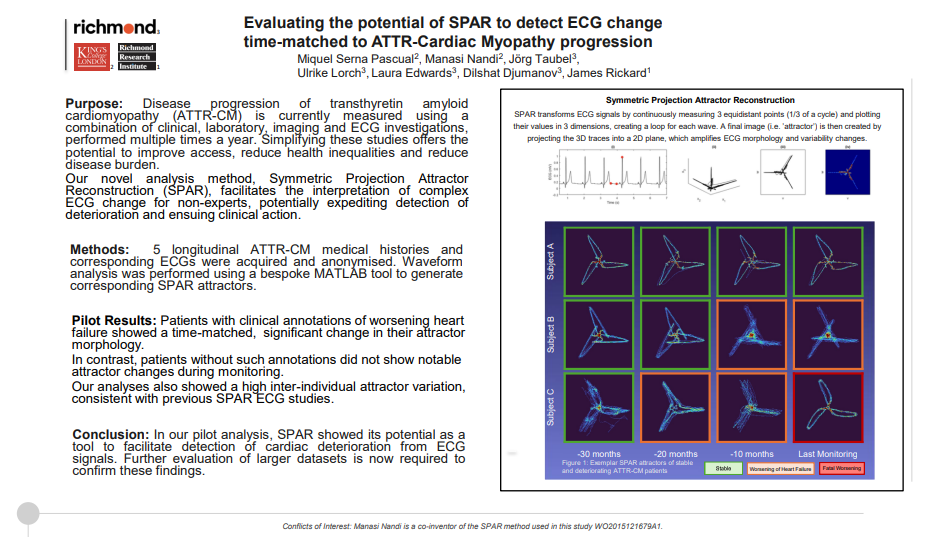
Evaluating the potential of SPAR to detect ECG change time-matched to ATTR-Cardiac Myopathy progression
July 17, 2025
View publication
Acoramidis-mediated early increase in serum transthyretin level reduces cardiovascular-related hospitalizations and mortality: insights from the attribute-cm study
July 17, 2025
View publication
Advanced Waveform Analysis of ECG Signals to Characterize Cardiac Drug Safety Between Caucasian & Japanese Volunteers - Poster 077
December 10, 2024
View publication
Advanced waveform analysis of the electrocardiogram signals using complementary signal processing techniques to investigate the response to a QTc prolonging drug vs control
November 12, 2024
View publication
Effect of Acoramidis on Myocardial Structure and Function in Transthyretin Amyloid Cardiomyopathy: Insights From the ATTRibute-CM Cardiac Magnetic Resonance(CMR) Substudy
November 12, 2024
View publication
Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy
September 13, 2024
View publication
Confirmation of the cardiac safety of nolasiban in a randomised cohort of healthy female volunteers
July 9, 2024
View publication
Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study
July 9, 2024
View publication
Haemodynamic effects of the nitroxyl donor cimlanod (BMS-986231) in chronic heart failure: a randomized trial
July 9, 2024
View publication
Comparing the consistency of electrocardiogram interval measurements by resting ECG versus 12-lead Holter
July 9, 2024
View publication
CRISPR-Cas9 in-vivo gene editing shows reduction in TTR after a single dose
June 29, 2021
View publication

Effects of progesterone and estradiol on QT subintervals over the course of a menstrual cycle
September 22, 2020
View publication
Do hERG blocking agents further increase the risk of sudden cardiac death in patients with type 1 diabetes?
September 22, 2020
View publication



Analyzing the Relationship of QT Interval and Exposure to Nitazoxanide
February 20, 2020
View publication




Comparison of Six Commonly Used QT Correction Models and Their Parameter Estimation Methods
February 20, 2020
View publication
Investigating the Ethnic Differences between the Effect of Moxifloxacin on Cardiac Conduction
February 20, 2020
View publication

Thorough QT study of the effect of oral moxifloxacin on QTc interval
February 20, 2020
View publication

Insulin at normal physiological levels does not prolong QTc interval
February 20, 2020
View publication

The Power Of Phase I Studies To Detect Clinically Relevant Qtc Prolongation
February 20, 2020
View publication
Investigating the effect of intravenous APD421 (Amilsulpride) and the ethnic differences
February 20, 2020
View publication
Confirmation of the Cardiac Safety of Rupatadine in a Single Ascending Dose and Multiple Ascending Dose
February 20, 2020
View publication



The Impact of Insulin Levels on QTc Interval in Thorough QT Studies
February 20, 2020
View publication


Alternative methods for the confirmation of assay sensitivity in Thorough QT studies
February 20, 2020
View publication





Cardiac Safety of Rupatadine in a Single-Ascending-Dose and Multiple-Ascending-Dose
February 20, 2020
View publication
Comparison of ECG Parameters Using GE Getemed Continuous ECG Holter and MAC-1200 Bedside ECG
February 20, 2020
View publication


A Phase I study to investigate the effects of Clascoterone on QT interval
February 20, 2020
View publication








Clinical Pharmacology and Adaptive Early Phase Studies

Phase 1 randomized double-blind study of an RNA interference therapeutic targeting HSD17B13 for metabolic dysfunction–associated steatohepatitis
July 17, 2025
View publication
A randomized, open‑label two‑period crossover pilot study to evaluate the relative bioavailability in the fed state of atovaquone‑proguanil (Atoguanil™) versus atovaquone‑proguanil hydrochloride (Malarone®) in healthy adult participants
July 10, 2024
View publication
The Value of In-Person Contact in Clinical Trials: A Qualitative Study examining Patient Attitudes towards Trial Visits conducted at a Contract Research Organisation
July 10, 2024
View publication
Association for Human Pharmacology in the Pharmaceutical Industry conference 2022: impending change, innovations and future challenges
July 10, 2024
View publication
Blinding Is Seeing: A Single-Centre Study Into the Viability of Auto-Injectors for Blinded-Drug Administration in Randomised Controlled Trials
July 10, 2024
View publication
Concentration-QT modelling of the novel DHFR inhibitor P218 in healthy male volunteers
July 10, 2024
View publication
Unanticipated CNS Safety Signal in a Placebo-Controlled, Randomized Trial of Co-Administered Atovaquone-Proguanil and Amodiaquine
July 10, 2024
View publication
Ethnic Sensitivity Study of the Extrafine, Single-Inhaler, Triple Therapy Beclomethasone Dipropionate, Formoterol Fumarate, and Glycopyrronium Bromide Pressurized Metered Dose Inhaler in Japanese and Caucasian Healthy Individuals
July 9, 2024
View publication
A Phase 1 Study to Investigate the Effects of Cortexolone 17α-Propionate, Also Known as Clascoterone, on the QT Interval Using the Meal Effect to Demonstrate ECG Assay Sensitivity
July 9, 2024
View publication


Transient paradoxical bronchospasm associated with inhalation of the LAMA AZD9164
February 20, 2020
View publication
The practical application of adaptive study design in early phase clinical trials
February 20, 2020
View publication
Rule Britannia – the advantages of conducting first-time-in-human (FTIH) studies in the UK
February 20, 2020
View publication
Cizolirtine Citrate (E-4018) in the Treatment of Chronic Neuropathic Pain
February 20, 2020
View publication
Pharmacokinetics, pharmacodynamics and safety of a human anti-IL-6 monoclonal antibody (sirukumab)
February 20, 2020
View publication

Ascending single-dose study with ACT-280778, a non-dihydropyridine, dual L- and T-type calcium channel blocker
February 20, 2020
View publication

The influence of a low fat diet and hospitalisation on liver function tests
February 20, 2020
View publication
A phase I, randomised single center, open-label parallel group trial to compare the pharmacokinetics
February 20, 2020
View publication



The pharmacokinetic interaction between isoniazid/pyrazinamide and TMC207
February 20, 2020
View publication


The Pharmacokinetic Interaction of the Selective PGF2α Receptor Antagonist OBE022
February 20, 2020
View publication
Pharmacokinetics and Safety of the Oral Prostaglandin F2 alpha Receptor Antagonist OBE022
February 20, 2020
View publication
Japanese Studies




Gastric Acid Suppression Effect of TAK-438, A Potassium-Competitive Acid Blocker
February 20, 2020
View publication

Gastric Acid Suppression Effect of TAK-438, A Potassium-Competitive Acid Blocker
February 20, 2020
View publication

The safety, tolerability and pharmacokinetics of AZD5069, a novel CXCR2 antagonist
February 20, 2020
View publicationPatient Studies

A Phase 2 study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of sutacimig for prophylaxis in glanzmann thrombasthenia: A trial in progress
December 12, 2025
View publication
Sutacimig, a novel bispecific antibody for prophylactic treatment of glanzmann thrombasthenia: Analysis of a Phase 2 study
December 12, 2025
View publication
Velora pioneer, Phase 1/2 study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of HMB-002 for prophylaxis in von Willebrand disease: A trial in progress
December 12, 2025
View publication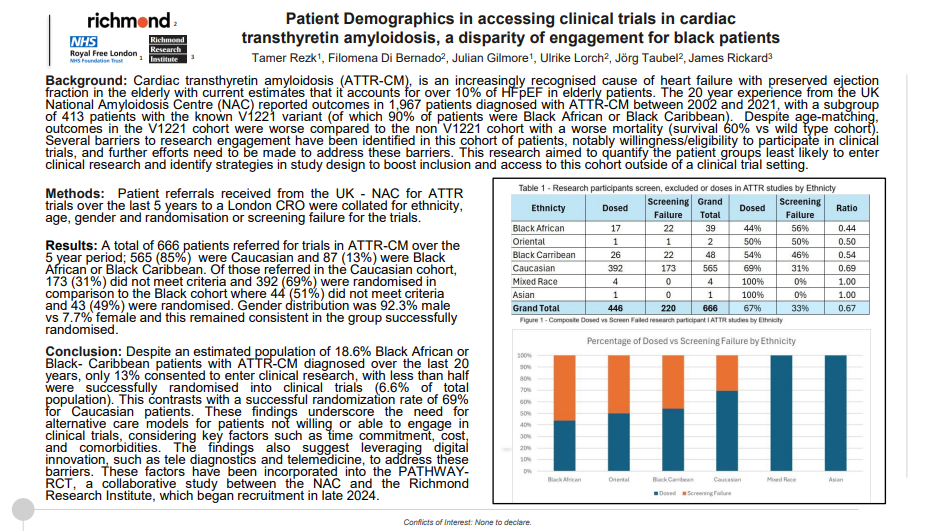
Patient Demographics in accessing clinical trials in cardiac transthyretin amyloidosis, a disparity of engagement for black patients
July 17, 2025
View publication
WED-435 Development of a novel model using machine learning algorithms to predict absence of metabolic associated steatotic liver disease in healthy and patient trial volunteers. A population screening tool
July 17, 2025
View publication
A Phase 1/2 Study to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of HMB-001 in Participants with Glanzmann Thrombasthenia
December 10, 2024
View publication
A first-in-human phase 1 study of cavrotolimod, a TLR9 agonist spherical nucleic acid, in healthy participants: Evidence of immune activation
July 10, 2024
View publication
Pharmacodynamic Consequences of Administration of VLA-4 Antagonist CDP323 to Multiple Sclerosis Subjects
February 20, 2020
View publication
Benefits of a Single Versus Multicenter Approach in Early-Phase Patient Studies
February 20, 2020
View publication


Efficacy and Safety of MED2005, a Topical Glyceryl Trinitrate Formulation, in the Treatment of Erectile Dysfunction
February 20, 2020
View publication


















.avif)
