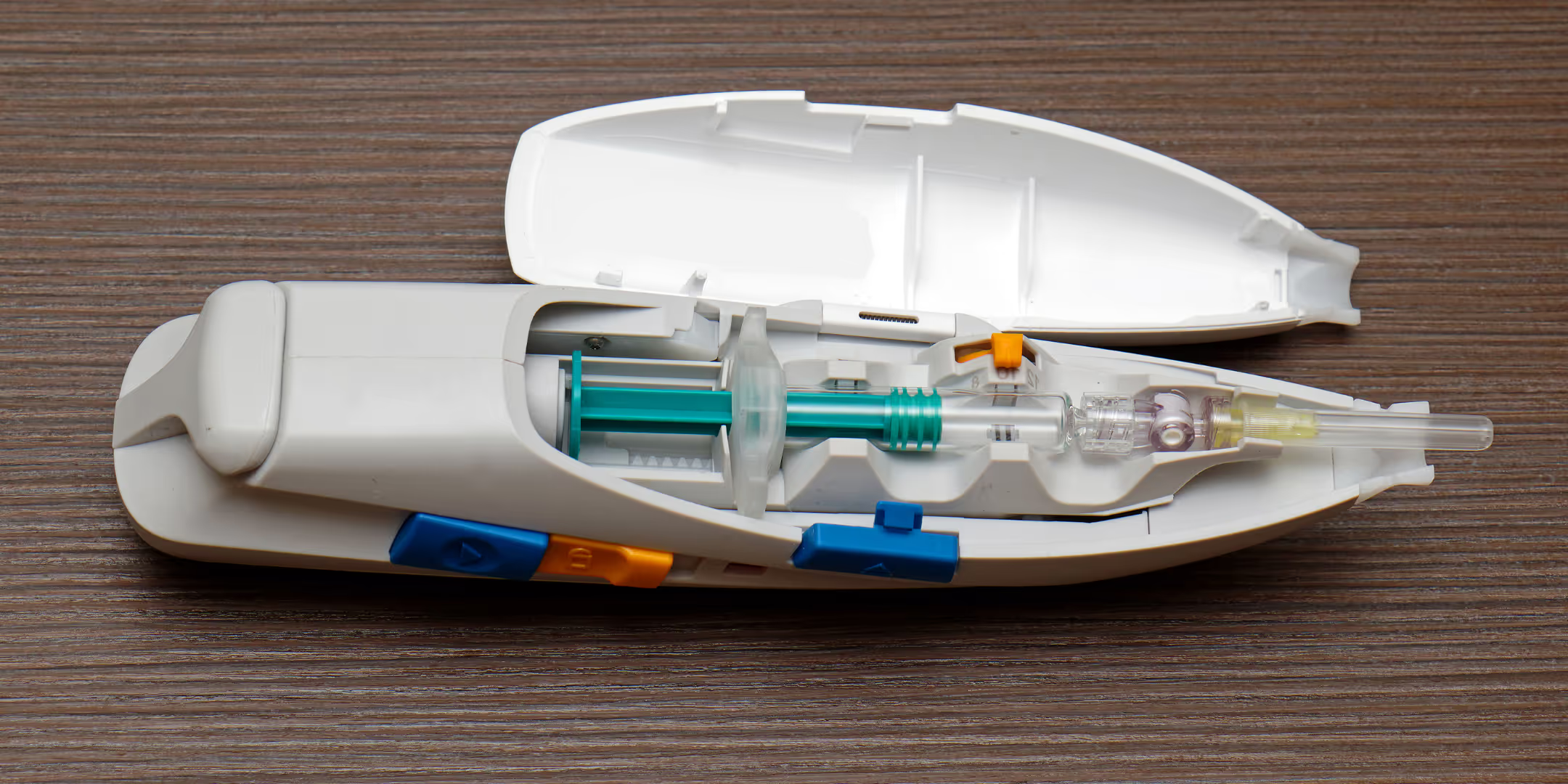
Maintaining investigator blinding in RCTs, especially when dealing with subcutaneous and intramuscular injections of investigational medicinal products (IMP), can be challenging.Discrepancies in the appearance and viscosity of active and placebo injections have posed a hurdle. Our study compared the performance of A-INJ (specifically, the Autoject 2) against conventional syringes (CS) for delivering 1 ml doses of both placebo and reference IMP.
The results demonstrated:
- No statistically significant differences in injectate delivery between A-INJ and CS.
- Investigators were largely unable to differentiate between the two solutions when using A-INJ, effectively maintaining blinding.
This suggested that A-INJ systems offer a promising solution for RCTs, eliminating the need to modify placebos with additives or colorants, or to utilise costly unblinded staff. These findings add to the growing body of evidence highlighting the advantages of A-INJs in administering injectable compounds during RCTs, opening up new possibilities for their utility in clinical research.















.avif)
