Regulatory strategy has long been an integral part of how we support development programmes.
Our Regulatory Services, led by Dr Lisa Campbell, formally set out this expertise as a defined offering, providing structured, proactive support across the regulatory lifecycle, from early planning through to approval.
Working closely with non-clinical, clinical, CMC, and commercial teams, we help clients navigate regulatory complexity with clarity, confidence, and purpose, providing a cross border, global lens.
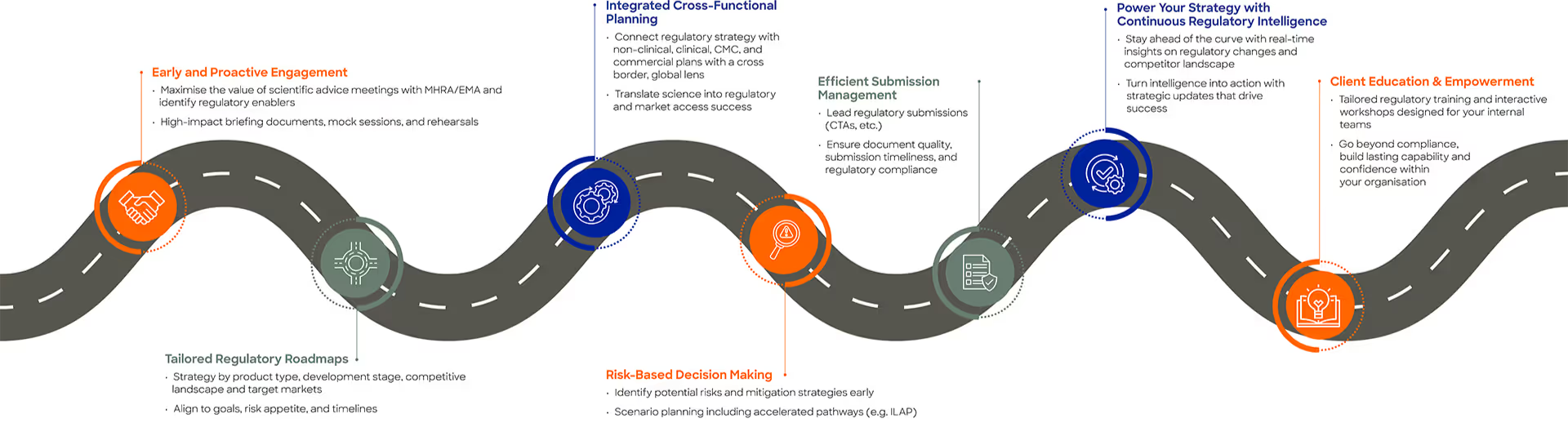
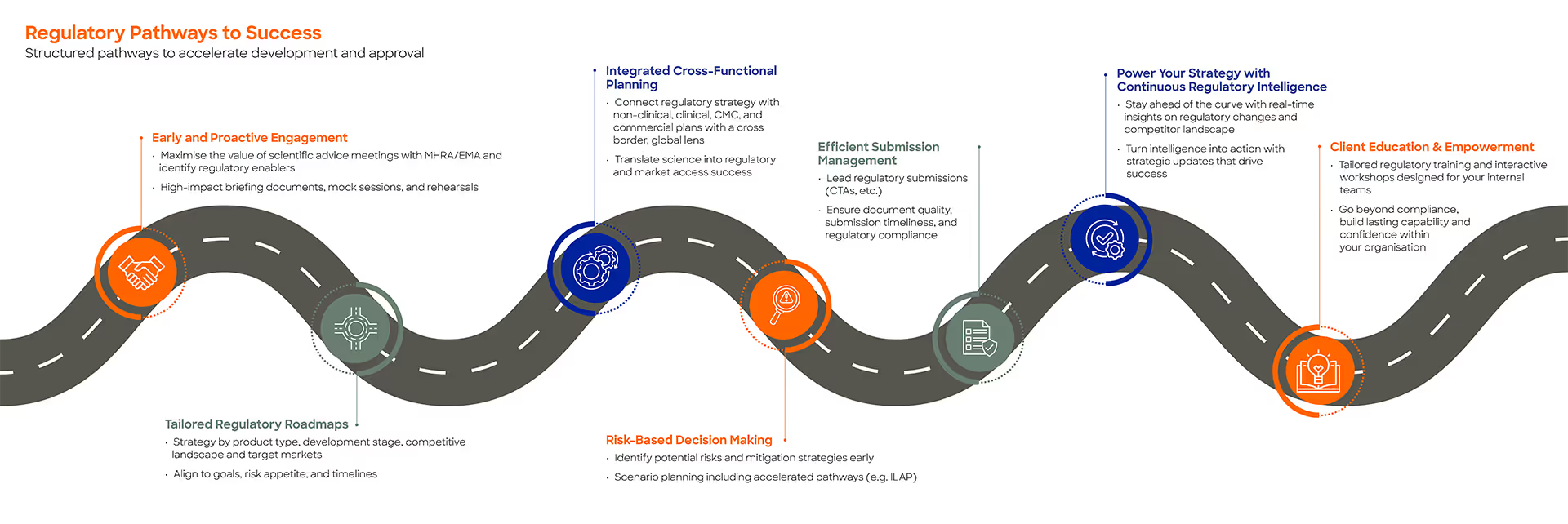
Our Regulatory Pathways to Success bring together:
- Early and proactive regulatory engagement, including scientific advice
- Tailored regulatory roadmaps aligned to product type, development stage, and target markets
- Integrated cross-functional planning across regulatory, non-clinical clinical, CMC, and commercial teams
- Risk-based decision-making, including accelerated and alternative pathways
- Efficient management of regulatory submissions
- Ongoing regulatory intelligence and strategic insight
- Client education and capability-building through bespoke training
The focus is on enabling better decisions throughout development, not simply achieving compliance at the point of submission.
Whether you’re shaping early development strategy or preparing for key regulatory milestones, our regulatory team can support your programme.
Email Faster Answers to discuss your development pathway.















.avif)
