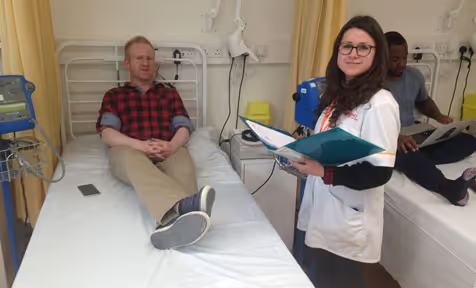
One month on from Richmond's opening event, which saw leading industry bodies and stakeholders convene, the CRO has been busy settling in their first patients at the new London Bridge site.Clinical work commenced on the 25th March with volunteers commenting on the excellent transport links and fantastic new location in the heart of London Bridge.
Latest news
Leadership Spotlight- Head of Acute Clinical Management
February 20, 2026
In this edition, we spotlight Carolina Pacheco de Amorim, who leads Acute Clinical Management (ACM)
Read moreEvents
Clinical Trials Innovation Programme
10 - 11 February 2026
Richmond Pharmacology is pleased to attend the Clinical Trials Innovation Programme Conference in Nice, France, 10–11 February 2026.
View event














.avif)
